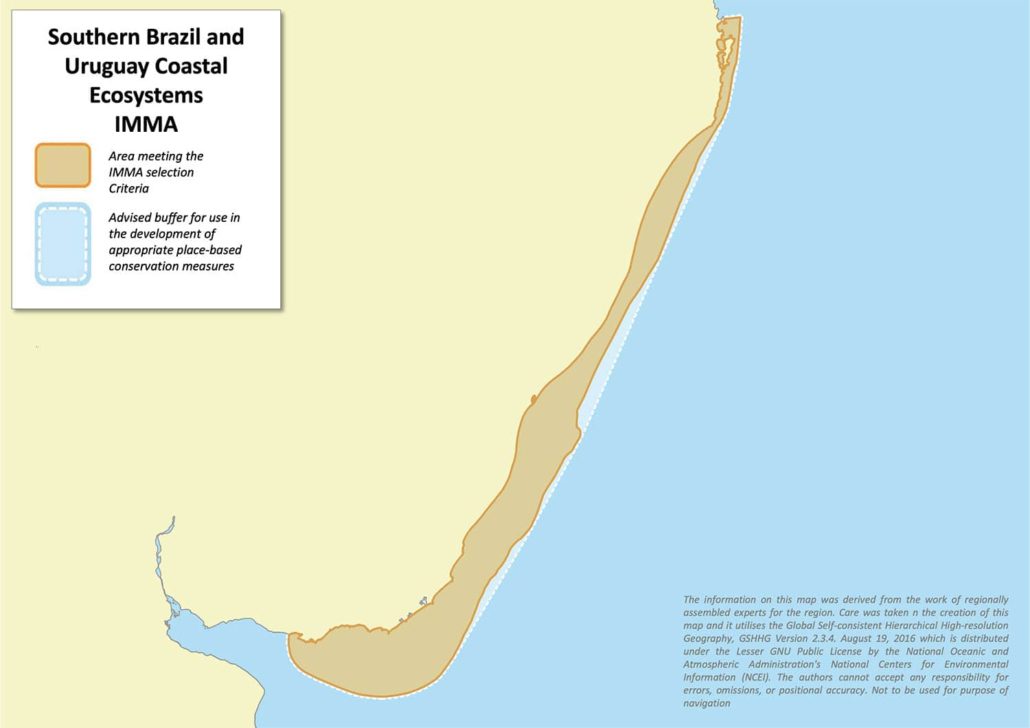
Southern Brazil and Uruguay Coastal Ecosystems IMMA
Size in Square Kilometres
66 550 km2
Qualifying Species and Criteria
Lahille’s bottlenose dolphins – Tursiops truncatus gephyreus
Criterion A; B (1)
Franciscana dolphin – Pontoporia blainvillei
Criterion A; D (1)
Southern right whale – Eubalaena australis
Criterion B (2); C (1)
South American sea lions – Otaria byronia
Criterion B (2); C (1,2,3)
South American fur seals – Arctocephalus australis
Criterion B (2); C (1,3)
Download fact sheet
Summary
The Southern Brazil and Uruguay Coastal Ecosystems IMMA comprises a mosaic of coastal habitats (from the shoreline out to the 50 m isobath, including inner estuaries, islands, rookeries and coastal bays) between Florianópolis, Brazil, in the north and the middle of the Rio de la Plata (political border) in the south where it extends westward up to the Santa Lucia river, Uruguay. It fully covers key habitats for two threatened cetaceans: the entire distributional range of Southern Brazil-Uruguay subpopulation of Lahille’s bottlenose dolphin and Franciscana Management Area III. The area also includes the second most important breeding ground for southern right whales in the Southwest Atlantic, as well as five main breeding colonies of South American fur seals and sea lions and associated feeding area of the latter over the continental shelf in one of the most productive oceanic regions of the world.
Description of Qualifying Criteria
Criterion A – Species or Population Vulnerability
Lahille’s bottlenose dolphin (Tursiops truncatus gephyreus) – The low abundance of the subspecies, with an estimate of fewer than 600 individuals alive in 2022, led to their classification as Vulnerable on the IUCN Red List (Vermeulen et al. 2019).
Franciscana (Pontoporia blainvillei) – As one of the most threatened small cetaceans in the western South Atlantic this species is listed as Vulnerable on the IUCN Red List of Threatened species, due to high bycatch and increasing habitat degradation throughout its range (Zerbini et al. 2017).
Criterion B: Distribution and Abundance
Sub-criterion B1: Small and Resident Populations
Lahille’s bottlenose dolphin – The Lahille’s bottlenose dolphin subspecies is divided into two geographically isolated subpopulations: Southern Brazil-Uruguay, and Argentina (Fruet et al. 2014). This IMMA covers the entire range of the Southern Brazil-Uruguay subpopulation, which consists of five small management units (Fruet et al. 2014). The Gephyreus Project – an international research initiative coordinating simultaneous photo-identification sampling at seven sites along the distribution range of the subspecies in order to investigate the metapopulation dynamics of the southern Brazil/Uruguay subpopulation – has been generating consistent results. Mark-recapture models in a Robust Design framework were fitted to data collected from the seven sampled sites in southern Brazil and Uruguay, collected during seven sampling periods between 2019 and 2022. These models estimated the number of marked individuals for each area per period. Total abundances, corrected by the mark rate estimation, ranged from 53 (95%CI: 50-57) to 62 (95%CI: 48-76) in Laguna; from 127 (95%CI: 80-174) to 252 (95%CI: 143-361) in Patos Lagoon; from 20 (95%CI: 11-30) to 29 (95%CI: 16-41) in Tramandaí; from 4 (95%CI: 3-8) to 47 (95%CI: 37-57) in Torres; from 8 (95%CI: 3-12) to 24 (95%CI: 4-12) in North Bay; from 10 (95%CI: 7-14) to 38 (95%CI: 21-55) in Uruguay. Combining the estimates from all areas, the total estimate for southern Brazil and Uruguay ranged from 246 (95%CI: 183-309) in the first period to 398 individuals (95%CI: 245-550) in the sixth sampling period, suggesting a total of < 400 individuals for the entire southern Brazil-Uruguay subpopulation (Fruet et al. 2023). High resighting rates of marked animals recorded within and between sampling periods suggests strong residence and site fidelity to estuaries and river mouths. Results from long-term mark-recapture studies with the resident local populations indicates that the region is used for reproduction and take care of young, with most births occurring during spring and summer, in association with increasing water temperature and food supply (see Fruet et al. 2016 for review; Fruet et al. 2015; Bezamat et al 2019). Inner waters of estuaries and bays are preferred for females to take care of their young. Interbirth-interval estimates are also available (Hoffman 2004; Fruet et al. 2015; 2016; Bezamat et al. 2019). For Baía Norte and Itajaí river, in southern Brazil, as well as in Uruguay, there are many records of the presence of mother and calf pairs using these areas constantly, but no reproductive rate estimation is available (Flores et al. 2007).
Sub-criterion B2: Aggregations
Southern right whale (Eubalaena australis) – The IMMA area encompasses the main wintering aggregation area of right whales along the Brazilian coast, from the central to southern coast of Santa Catarina state (Renault-Braga et al. 2021), designated by the Federal Government of Brazil as a Marine Protected Area dedicated to the species recovery and conservation (Southern Right Whale Environmental Protected Area). The area is a major wintering ground for a threatened population that was greatly depleted by whaling. The Southwestern Atlantic population is currently estimated to be less than 10% of its original numbers (Romero et al. 2022).
South American fur seals (Arctocephalus australis) and sea lions (Otaria byronia) – These two species are distributed in the Pacific and the Atlantic Oceans (Vaz-Ferreira 1982a,b), and just the Uruguayan and Brazilian populations belong to this IMMA. Both species show a patchy distribution of breeding colonies and resting sites in both sides of South America (Tunez et al. 2008, Crespo & Oliveira, 2021; Crespo et al. 2021). Both species occupy five Uruguayan breeding colonies (Franco-Trecu et al., 2019) and two Brazilian marine protected areas (Procksch et al. 2020). The total (uncorrected) South American fur seal abundance in Uruguay from aerial photos in 2013 was 45,588 (SD = 1,536) individuals, 67% of which were at Punta del Este (Isla – Islote de Lobos). In 2013, the corrected pup abundance estimate was 12,741 for Cabo Polonio islands and 18,419 for Isla – Islote de Lobos (Franco-Trecu et al., 2019). For the southern Brazilian coast, the observed numbers in the last decade for both species of the South American pinnipeds in the two wildlife refuges ranged from 300 to 500 individuals per year (Pavanato et al. 2013; Procksch et al. 2020).
The total (uncorrected) South American fur seal abundance in Uruguay from aerial photos in 2013 was 45,588 (SD = 1,536) individuals, 67% of which were at Punta del Este (Isla – Islote de Lobos). In 2013, the corrected pup abundance estimate was 12,741 for Cabo Polonio islands and 18,419 for Isla – Islote de Lobos (Franco-Trecu et al., 2019). For the southern Brazilian coast, the observed numbers in the last decade for both species of South American pinnipeds in the two wildlife refuges ranged from 300 to 500 individuals per year (Pavanato et al., 2013; Procksch et al., 2020).
Criterion C: Key Life Cycle Activities
Sub-criterion C1: Reproductive Areas
Southern right whales – The southern right whale which underwent a dramatic demographic bottleneck due to whaling, has a historical and contemporary circumpolar distribution from around 12°S to 65°S, albeit with a discontinuity between Chile and New Zealand. In the Atlantic coast of South America, southern right whales aggregate in two main breeding grounds: Peninsula Valdes, Argentina, and the bays of central-southern Santa Catarina State, southern Brazil. The whales use these areas mainly for calving, resting and reproduction. Recent genetic and mark-recapture studies showed connectivity between Brazil and Argentina, exemplified by weak genetic differentiation and the movement of genetically and naturally identified individuals between the Western South Atlantic grounds (Ott et al 2011, Rowntree et al 2020). In Uruguay, coastal areas such as Punta del Este host congregating sites for whales in breeding seasons, but these are not likely to be calving grounds (Costa et al 2007). Right whales have been monitored since the 1980’s along the southern Brazilian coast. Most groups sighted in this region are mother-calf pairs with a few sightings of juveniles, single adults and mating groups (Seyboth et al. 2015, Danilewicz et al, 2016; Renault-Braga et al. 2018, 2021a). In this area, female right whales aggregate for about five months of the winter (from July to November), and early spring breeding season. The area has bays which protect the species from strong winds and waves, important for animals to give birth, and/or care for young during their first months of life (e.g., Groch et al. 2005; Seyboth et al. 2015; Renault-Braga et al. 2018). As the population increases there is increasing occurrence of the species south of Santa Catarina (Danilewicz et al, 2016), in an area likely used for mating (Renault-Braga et al. 2021b, 2022). The Brazilian Right Whale Catalogue includes more than 1000 photo-identified whales. The most recent estimate of population growth rate in Brazil was 4.8%/year with an abundance estimate of 569 mature females (Renault-Braga et al. 2021b).
South American fur seals – Reproductive areas for fur seals within this IMMA were reported for the Uruguayan coastline. The breeding colonies for South American fur seals are: Lobos’ Island and islet (35º 01′ S, 54º 52′ W), Torres group Islands (34º 21′ S, 53º 44′ W) which includes Rasa (0.03 km2), Encantada (0.02 km2), and Islote (0.02 km2), together with Del Marco Island (0.08 km2) (34º 25′ S, 53º 46′ W) which is located ca. 2 km offshore and within the Cabo Polonio National Park that was established in 2009 (Franco-Trecu et al., 2019). The biggest breeding colony of South American fur seals is Isla de Lobos, Uruguay (Franco-Trecu et al., 2019).
South American sea lions – Reproductive areas for sea lions within this IMMA were reported for the Uruguayan coastline only. Their main breeding colonies are two groups of rookeries: Lobos’ Island and islet and the group of islands of Torres-Del Marco. The northernmost breeding colony of this species in the Atlantic is also located in Uruguay, at the Verde Island and Coronilla Island (33°56’S 53°29’W; Vaz-Ferreira 1975), where the reproductive activity is low with a few numbers of births recorded (Szteren, 2015). The latter are part of the Isla Verde and Islas de la Coronilla’s Habitat and/or Species Management Area.
Sub-criterion C2: Feeding Areas
South American sea lions – The South American sea lions have a very broad diet within the Atlantic Ocean. The foraging habitat specially covers an extensive area in the Río de la Plata estuary and its plume, with a clear center of activity around Lobos’ Island and a significant overlap between subadults and adults (Rodríguez et al., 2013). Moreover, in waters of Rio de la Plata and the adjacent Atlantic Ocean, two important prey items of sea lions’ diet, the stripped weakfish (Cynoscion guatucupa) and the whitemouth croaker (Micropogonias furnieri) are also targeted by both the artisanal fisheries and the coastal bottom trawl fisheries operating in the area, and interactions between fisheries and sea lions’ have been reported (Szteren & Paez, 2002; Franco-Trecu et al., 2019).
Lactating South American sea lions are central place-foragers (Orians and Pearson, 1977) that make foraging trips at sea and return to their rookeries or colony (central place) to nurse their young (Thompson et al., 1998). As a consequence, the latter behaviour and foraging are separated in time and space (Bonner, 1984); therefore, it is crucial that foraging areas be close to the rookeries since travelling to these sites can incur high energy cost. This is particularly important for lactating pinniped females that must forage during lactation (Boness et al., 1994).
Based on foraging behaviour-satellite-tracking data from individuals tagged in Lobos’ Island breeding colony (35°01’S; 54°52’W), it was shown that lactating South American sea lions are benthic divers and that they forage in shallow waters within the continental shelf (Riet-Sapriza et al., 2013). Most of the tracked dives (70%) occurred between 15 and 25 m depth, followed by the depth range of 5 – 10 m (23%), and a few dives (7%) between the 30 and 55 m depth (Riet-Sapriza et al., 2013). The maximum distance travelled on a foraging trip from Lobos’ Island ranged between 37.8 and 135.5 km.
Sub-criterion C3: Migration Routes
South American fur seals and sea lions – Every year hundreds of pinnipeds of various species are found along the southern Brazilian coast, most of which are South American fur seals and South American sea lions (Pinedo 1990; Rosas et al. 1994; Simões-Lopes et al. 1995, Oliveira 2013). Brazil has no breeding colonies for any species of pinniped and only two haulout sites that are within marine protected areas (MPAs): the Wildlife Refuge of Molhe Leste of São José de Norte (WRML) (32° 10′ S; 52° 06′ W), and the Wildlife Refuge of Ilha dos Lobos (WRIL) (29° 20′ S; 49° 42′ W), both on the coast of the Rio Grande do Sul, southern Brazil (Oliveira 2013). The arrival of these species in southern Brazil usually corresponds to the dispersal period after the breeding season, which occurs from December to February, and may be assisted by the cold, northwards following Malvinas Current (Pinedo 1990; Rosas et al. 1994; Simões-Lopes 1995., Oliveira 2013). This dispersal period towards Brazilian waters is one of the least understood periods of the life-cycle of South American pinnipeds (Bastida & Rodríguez, 1994; Sanfelice et al., 1999). Recent tagging (Giardino et al., 2014) and genetic studies (Oliveira et al., 2008a, 2017) have confirmed that South American fur seals and South American sea lions from Argentine and Uruguayan colonies disperse northwards to Brazil.
Criterion D: Special Attributes
Sub-criterion D1: Distinctiveness
Lahille’s bottlenose dolphin – Fruet et al. (2014) performed an analysis combining 16 microsatellite loci and mitochondrial DNA (mtDNA) control region sequences in 124 biopsy samples collected over six local populations of photographically identified Lahille’s bottlenose dolphins in southern Brazil, Uruguay and central Argentina. Results revealed remarkably low levels of genetic diversity and strong genetic differentiation between dolphins from southern Brazil–Uruguay (SB–U) and Bahía San Antonio (BSA), Argentina. Negligible contemporary gene flow between units was estimated based on Bayesian analysis, suggesting they should be considered two distinct evolutionarily significant units. Results of this paper were discussed during the Second International Workshop on Research and Conservation of Tursiops in the Southwest Atlantic Ocean“, held in Cassino Beach, Rio Grande, Brazil, from 6 to 8 April 2017. Participants recognized the two main units proposed by Fruet et al. (2014): Southern Brazil-Uruguay (SB-U) and Argentina. After discussion, participants agreed that they are genetically and ecologically different.
In addition, it is also important to consider the cooperative fishing behavior between the Lahille’s bottlenose dolphins and the castnet fishers. This unique feeding behavioural tactic is restricted to a few local populations of Lahille’s bottlenose dolphins in southern Brazil – considered intangible natural heritage (Simões-Lopes et al. 2016). Specific studies on such behavior support evidence in favor of the cultural transmission of the cooperative foraging tactic among dolphins: stereotyped and synchronized behaviors, which are shared among subsets of the population and maintained across generations via social learning, primarily from mothers to calves (Simões-Lopes et al. 2016; van der Wal et al. 2022).
Supporting Information
Bassoi, M., Shepherd, J.G., Secchi, E.R., Moreno, I.B., Danilewicz, D. 2020. ‘Oceanographic processes driving the feeding ecology of franciscana dolphin off Southern Brazilian coast’. Continental Shelf Research, 201: 104–124.
Bastida, R. & Rodríguez, D. (1994). Hallazgo de un apostadero estacional de lobos marinos de dos pelos, Arctocephalus australis (Zimmermann, 1783), en bajos fondos frente a la costa de Mar del Plata (Provincia de Buenos Aires, Argentina). Anales 4ª Reunión de Trabajo de Especialistas en Mamíferos Acuáticos de América del Sur 1–22.
Bonner, W.N., 1984. Lactation strategies in pinnipeds: problems for a marine mammalian group. Symp. Zool. Soc. Lond. 51, 253–272.
Boness DJ, Bowen WD, Oftedal OT (1994) Evidence of a maternal foraging cycle resembling that of otariid seals in a small phocid, the harbor seal. Behav Ecol Sociobiol 34:95–104.
Botta, S. Bassoi, M. and Troina, G.C. 2022. Overview of franciscana diet. pp. 14-48. In: Simões-Lopes, P.C. and Cremer, M.J. (Eds). The Franciscana Dolphin. Academic Press.
Burrage, D., Wesson, J., Martinez, C., Pérez, T., Moller, O. and Piola, A. 2008. “Patos Lagoon outflow within the Río de la Plata plume using an airborne salinity mapper: observing an embedded plume”. Continental Shelf Research, 28:1625–1638. https://doi.org/10.1016/j.csr.2007.02.014
Cooke, J.G. and Zerbini, A.N. 2018. Eubalaena australis. IUCN Red List of Threatened Species 2018: e. T8153A50354147.
Costa-Urrutia, P., Piedra, M., Franco-Fraguas, P. and Paez, E. 2007. “Distribution and habitat use patterns of southern right whales, Eubalaena australis, off Uruguay”. Journal of Cetacean Research Management 9(1): 45-51.
Crespo E.A. & Oliveira L.R. (2021). South American fur seal Arctocephalus australis, (Zimmerman 1783). In: Ecology and Conservation of Pinnipeds in Latin America (Heckel G. & Schramm Y. Eds). 13-30pp.
Crespo E.A., Oliveira L.R.& Sepúlveda M. (2021). South American sea lion (Otaria flavescens, Shaw 1800) In: Ecology and Conservation of Pinnipeds in Latin America (Heckel G. & Schramm Y. Eds). 93-118pp.
Cunha, H.A., Medeiros, B.V., Barbosa, L.A., Cremer, M.J., Marigo, J., Lailson- Brito, J., et al. 2014. ‘Population structure of the endangered franciscana dolphin (Pontoporia blainvillei): reassessing management units’. PLoS One 9:e85633.Available at https://doi.org/10.1371/journal.pone.0085633
Danilewicks, D., Moreno, I.B., Tavares, M. and Sucunza, F. 2016. Southern right whales (Eubalaena australis) off Torres, Brazil: Group characteristics, movements, and insights into the role of the Brazilian‐Uruguayan wintering ground. Mammalia, 81(3): 225–234.
Danilewicz, D.S., Secchi, E.R., Ott, P.H., Moreno, I.B., Bassoi, M. and Martins, M.B. 2009. ‘Habitat use patterns of franciscana dolphin (Pontoporia blainvillei) off southern Brazil in relation to water depth’. Journal of Marine Biological Association of the United Kingdom 89: 943–949. Avalilable at https://doi.org/10.1017/s002531540900054x
Danilewicz, D., Moreno, I.B., Ott, P.H., Tavares, M., Azevedo, A.F., Secchi, E.R., et al. 2010. ‘Abundance estimate for a threatened population of franciscana dolphins in southern coastal Brazil: uncertainties and management implications’. Journal of Marine Biological Association of the United Kingdom 90: 1649–1657. Available at https://doi.org/10.1017/s0025315409991482
Franco-Trecu, V., Massimiliano, D., Grandi, M.F., Soutullo, A., Crespo, E.A. and Inchausti, P. 2019. “Abundance and Population Trends of the South American Fur Seal (Arctocephalus australis) in Uruguay”. Aquatic Mammals 45(1): 48-55. Available at https://doi.org/10.1578/AM.45.1.2019.48
Fruet, P.F., Daura-Jorge, F.G., Genoves, R.C., Castilho, P.V., Laporta, P., Di Tullio, J.C., Vermeulen, E., Berninsone, L., Coscarella, M., Failla, M. and Iniguez, M. 2020. “Lahille’s bottlenose dolphins: conservation status update, working in progress and follow up on the previous SC66b/SM recommendations”. Document SC/68B/SM11 presented during the Scientific Committee Meeting of the International Whaling Commission, Cambridge.
Fruet, P.F., Daura-Jorge F.G., Laporta, P., Coscarella, M., Vermeulen, E., Ott, P.H., Berninsone, L., Genoves, R.C., Moreno, I.B., Failla, M., Flores, P.A.C., Iniguez, M., Pretto, D.J., Machado, R., Perez, M.S., Bezamat, C., Secchi, E.R., Castilho, P.V., Barreto, A.S., Carrion, M., Frainer, G. and Di Tullio, J.C. 2023. “Progress report on the research and conservation of Lahille’s bottlenose dolphins – 2022”. Document SC/69A/SM05 presented during the Scientific Committee Meeting of the International Whaling Commission, Bled, Slovenia.
Fruet, P.F, Laporta, P. and Flores, P.A. 2016. “Report of the working group on population parameters and demography of Tursiops truncatus in the southwest Atlantic Ocean”. The Latin American Journal of Aquatic Mammals 11(1-2): 71-78. Available at http://dx.doi.org/10.5597/lajam00217
Fruet, P.F., Daura-Jorge, F.G., Moller, L.M., Genoves, R.C. and Secchi, E.R. 2015. Abundance and demography of bottlenose dolphins inhabiting a subtropical estuary in the Southwestern Atlantic Ocean. Journal of Mammalogy 96: 332 – 343.
Fruet, P.F., Secchi, E.R., Daura-Jorge, F.G, Vermeulen, E., Flores, P.A.C., Simões-Lopes, P.C., Genoves, R.C., Laporta, P., Di Tullio, J.C., Freitas, T.R.O., Dalla Rosa, L., Valiati, V.H., Beheregaray, L.B. and Möller, L.M. 2014. “Remarkably low genetic diversity and strong population structure in common bottlenose dolphins (Tursiops truncatus) from coastal waters of the Southwestern Atlantic Ocean”. Conservation Genetics 15: 879 – 895.
Groch, K., Palazzo, J.T., Flores, P., Ardler, F. and Fabian, M. 2005. Recent rapid increase in the right whale (Eubalaena australis) population off southern Brazil. Latin American Journal of Aquatic Mammals 4:41–47.
ICMBio. (2018). Livro Vermelho da Fauna Brasileira Ameaçada de Extinção volume ii – mamíferos. Available at https://www.icmbio.gov.br/portal/images/stories/comunicacao/publicacoes/publicacoes-diversas/livro_vermelho_2018_vol2.pdf
Laporta, P., Martins, C., Lodi, L., Domit, C., Vermeulen, E. and Di Tullio, J.C. 2016. “Report of the Working Group on Habitat Use of Tursiops truncatus in the Southwest Atlantic Ocean”. Latin American Journal of Aquatic Mammals 11(1-2): 47-61.
Laporta, P., Fruet, P.F. and Secchi, E.R. 2016. “First estimate of common bottlenose dolphin (Tursiops truncatus) (Cetacea, Delphinidae) abundance off Uruguayan Atlantic coast”. The Latin American Journal of Aquatic Mammals 11(1-2): 144-154. Available at http://dx.doi.org/10.5597/lajam00223
Ministério do Meio Ambiente. 2022. PORTARIA MMA Nº 148, DE 7 DE JUNHO DE 2022. Available at https://in.gov.br/en/web/dou/-/portaria-mma-n-148-de-7-de-junho-de-2022-406272733
Moller, O.O., Piola, A.R., Freitas, A.C. and Campos, E.J. 2008. “The effects of river discharge and seasonal winds on the shelf off southeastern South America”. Continental Shelf Research 28:1607–1624. https://doi.org/10.1016/j.csr.2008.03.012
Oliveira LR, Hoffman JI, Hingst-Zaher E. et al. (2008a); Morphological and genetic evidence for two evolutionarily significant units (ESUs) in the South American fur seal, Arctocephalus australis. Conserv Genet 9:1451–1466.
Oliveira, L.R. Carnívoros marinhos in Mamíferos do Rio Grande do Sul. (2013). (Eds. Weber, M.M., Roman, C. & Cáceres, N.C.) 405-227 (Editora UFSM).
Oliveira, L. R. et al. (2017). Ancient female philopatry, asymmetric male gene flow, and synchronous population expansion support the influence of climatic oscillations on the evolution of South American sea lion (Otaria flavescens). PLoS ONE 12, e0179442.
Orians, G.H., Pearson, N.E., 1977. On the theory of central place foraging. In: Horn, D.J., Stairs, G.R., Mitchell, R.D. (Eds.), Analysis of Ecological Systems. Ohio State University Press, Columbus, OH, pp. 153–177.
Ott, P.H., Flores, P.A.C., Freitas, T.R.O. and White, B.N. 2011. “Genetic diversity and population structure of southern right whales, Eubalaena australis, from the Atlantic coast of South America”. Report SC/12/RW25 presented to the Scientific Committee of the International Whaling Commission. Cambridge (UK): Scientific Committee of the IWC. Available from: https://iwc.int/home
Palma, E.D., Matano, R.P. and Piola, A.R. 2004. “A numerical study of the Southwestern Atlantic Shelf circulation: barotropic response to tidal and wind forcing”. Journal of Geophysical Research 109:C08014. Available at https://doi.org/10.1029/2004jc002315
Palma, E.D., Matano, R.P. and Piola, A.R. 2008. “A numerical study of the Southwestern Atlantic Shelf circulation: stratified ocean response to local and offshore forcing”. J Geophys Res 113:C11010. https://doi.org/10.1029/2007jc004720
Pavanato H., Silva K.G., Estima S.C., Monteiro D.S. and Kinas P.G. (2013). Occupancy dynamics of South American Sea-Lions in Brazilian haul-outs. Brazilian Journal of Biology 73, 855-862.
Pezzi, L.P., Souza, R.B., Farias, P.C., Acevedo, O. and Miller, A.J. 2016. “Air-sea interaction at the Southern Brazilian Continental Shelf: in situ observations”. Journal of Geophysical Research 121:6671–6695. Available at https://doi.org/10.1002/2016jc011774
Piola, A.R. 2005. The influence of the Plata River discharge on the western South Atlantic shelf. Journal of Geophysical Research 32:L01603. Available at https://doi.org/10.1029/2004gl021638
Piola, A.R. and Matano, R.P. 2001. “Brazil and Falklands (Malvinas) currents”. In: Steele, J.H. (ed) Encyclopedia of ocean sciences. Academic Press, Oxford, pp 340–349. Available at https://doi.org/10.1006/rwos.2001.0358
Pinedo, M.C. Ocorrência de pinípedes na costa brasileira. Garcia de Orta Serie de Zoologia 15, 37–48 (1990).
Prado, J.H.F., Kinas, P.G., Pennino, M.G., Seyboth, E., Silveira, F.R.G., Ferreira, E.C. et al. 2021. ‘Definition of no-fishing zones and fishing effort limits to reduce franciscana bycatch to sustainable levels in southern Brazil’. Animal Conservation. https://doi.org/10.1111/acv.12679
Procksch, N. et al. 2020. “The northernmost haulout site of South American sea lions and fur seals in the western South Atlantic”. Scientific Reports 10.
Renault-Braga, E.P., Groch, K.R., Flores, P.A.C. et al. 2018. ‘Area usage estimation and spatiotemporal variability in distribution patterns of southern right whales, Eubalaena australis, in southern Brazil’. Marine Ecology 39: e12506. Available https://doi.org/10.1111/maec.12506
Renault-Braga, E.P., Groch, K.R., Simões-Lopes, P.C. 2021a. ‘Is there spatial segregation between reproductive groups of southern right whales along the coastline of southern Brazil?’ Marine Mammal Science. 10.1111/mms.12797. Available at https://doi.org/10.1111/mms.12797
Renault-Braga, E.P., Groch, K.R. and Simões-Lopes, P.C. 2021b. ‘Numerical population estimates update for Southern Right Whales in Brazil’. Report of the International Whaling Commission. Document SC/68C/CMP/10.
Riet-Sapriza F.G., Costa D.P., Franco-Trecu V., Marín Y., Chocca J., González B., … & Hückstadt L. A. (2013). Foraging behavior of lactating South American sea lions (Otaria flavescens) and spatial–temporal resource overlap with the Uruguayan fisheries. Deep Sea Research Part II: Topical Studies in Oceanography, 88, 106-119.
Riet-Sapriza, F., Jimenez, E., Jorge, G. and Costa, P. 2011. Utilization distribution of southern right whales Eubalaena australis off the coast of Uruguay. Paper SC/S11/RW9 presented to the Southern Right Whale Assessment Workshop, 13–16 September 2011, Buenos Aires, Argentina (unpublished). 10pp.
Rodríguez D.H, Dassis M., Ponce de León A., Barreiro C, Farenga M, Bastida R.O. Davis R.W. (2013). Foraging strategies of Southern sea lion females in the La Plata River Estuary (Argentina–Uruguay) Deep-Sea Research II 88–89 120–130.
Romero, M.A., Coscarella, M.A., Adams, G.D., Pedraza, J.C., González, R.A. and Crespo E.A. 2022. ‘Historical reconstruction of the population dynamics of southern right whales in the southwestern Atlantic Ocean’. Scientific Reports 12(1):3324. Available at https://doi.org/10.1038/s41598-022-07370-6.
Rosas, F. C. W., Pinedo, M. C., Marmotel, M. & Haimovici, M. 1994. Seasonal movements of the South American sea lion (Otaria flavescens Shaw, 1800) of the Rio Grande do Sul coast Brazil. Mammalia 58, 51–59.
Rowntree, V., Groch, K.R., Vilches, F. and Sironi, M. 2020. ‘Sighting histories of 124 southern right whales recorded of both southern Brazil and Peninsula Valdes, Argentina, between 1971 and 2017’. Report International Whaling Commission, document SC/68B/CMP/20.
Sanfelice, D., Vasques, V. C. & Crespo, E. A. 1999. Ocupação sazonal por duas espécies de Otariidae (Mammalia, Carnivora) da Reserva Ecológica Ilha dos Lobos, Rio Grande do Sul, sul do Brasil. Iheringia, Sér. Zool. 87, 101–110.
Secchi, E.R., Cremer, M.J., Danilewicz, D. and Lailson-Brito, J. 2021. ‘A Synthesis of the Ecology, Human-Related Threats and Conservation Perspectives for the Endangered Franciscana Dolphin’. Frontiers in Marine Science 8:617956. Available at https://doi.org/10.3389/fmars.2021.617956
Secchi, E.R., Danilewicz, D. and Ott, P.H. 2003a. ‘Applying the phylogeographic concept to identify franciscana dolphin stocks: implications to meet management objectives’. Journal of Cetacean Research and Management 5 : 61–68.
Secchi, E.R., Danilewicz, D. and Ott, P.H. (2003a). “Applying the phylogeographic concept to identify franciscana dolphin stocks: implications to meet management objectives”. Journal of Cetacean Research and Management 5: 61–68.
Secchi, E.R., Ott, P.H., Crespo, E.A., Kinas, P.G., Pedraza, S.N. and Bordino, P. 2001. “A first estimate of franciscana (Pontoporia blainvillei) abundance off southern Brazil”. Journal of Cetacean Research and Management 3: 95–100.
Secretariat of the Convention on Biological Diversity 2014. Ecologically or Biologically Significant
Marine Areas (EBSAs): Special places in the world’s oceans. Volume 2: Wider Caribbean and Western Mid-Atlantic Region. 86 pages
Seyboth E., Groch K.R., Secchi E.R. and Dalla-Rosa L. 2015. ‘Habitat use by southern right whales, Eubalaena australis Desmoulins, 1822, in their main northern calving area in the western South Atlantic’. Marine Mammal Science 314: 1521-1537.
Simões-Lopes, P. C., Drehmer, C. J. & Ott, P. H. (1995). Nota sobre os Otariidae e Phocidae (Mammalia: Carnivora) da costa norte do Rio Grande do Sul e Santa Catarina Brasil. Biociências 3, 173–181.
Soares, I.D., Kourafalou, V. and Lee, T.N. 2007. “Circulation on the western South Atlantic continental shelf: Spring and autumn realistic simulations. Journal of Geophysical Research 112:C04003. Available at https://doi.org/10.1029/2006JC003620
Szteren, D. (2015). Otaria favescens and Arctocephaus australis abundance in poorly known sites: a spatial expansion of colonies?. Braz. J. Oceanogr. 63, 337–346.
Szteren D. and Paéz E. 2002. Predation by southern sea lions (Otaria flavescens) on artisanal fishing catches in Uruguay. Mar. Freshwater Res. 53(8): 1161-1167.
Stramma, L., Ikeda, Y. and Peterson, R.G. 1990. “Geostrophic transport in the Brazil current region north of 20◦S”. Deep Sea Reearch Part A 37:1875–1886. Available at https://doi.org/10.1016/0198-0149(90)90083-8
Szephegyi, M.N. 2012. “Captura incidental y uso de hábitat del delfín franciscana (Pontoporia blainvillei) en el Río de la Plata y la costa atlántica uruguaya a partir de información de las flotas pesqueras”. Master’s thesis, Facultad de Ciencias, PEDECIBA BIOLOGÍA- Subárea Ecología, Universidad de la República.
Thompson D., Duck C.D., McConnell B.J., Garett, J. (1998). Foraging behavior and diet of lactating female southern sea lions (Otaria flavescens) in the Falkland Islands. J. Zool. 246, 135–146.
Túnez J.I., Cappozzo H.L., Cassini M.H. (2008). Regional factors associated with the distribution of South American fur seals along the Atlantic coast of South America. ICES J Mar Sci 65:1733–1738.
van derWal, J. E. M., Spottiswoode, C. N., Uomini, N. T., Cantor, M., Daura-Jorge, F. G., Afan, A. I., Attwood, M. C., Amphaeris, J., Balasani, F., Begg, C. M., Blair, C. J., Bronstein, J. L., Buanachique, I. O., Cuthill, R. R. T., Das, J., Deb, A., Dixit, T., Dlamini, G. S., Dounias, E., … Cram, D. L.. Safeguarding human–wildlife cooperation. Conservation Letters. 2022;15:e12886. https://doi.org/10.1111/conl.12886
Vaz Ferreira R. (1975). Factors affecting numbers of sea lions and fur seals on the Uruguayan islands. Rapp. P-V. Réunion du Conseil International pour Exploration de la Mer 169:257-262
Vaz Ferreira R. (1982a). Arctocephalus australis (Zimmermann), South American Fur Seal. Pp. 497-508. In: Mammals in the Seas. FAO Fisheries Series 5, Volume 4. Small cetaceans, seals, sirenias and otters. 531 pp.
Vaz Ferreira R (1982b). Otaria flavescens (Shaw), South American Sea Lion. Pp 477-495 In: Mammals in the Seas. FAO Fisheries Series 5, Volume 4. Small cetaceans, seals, sirenias and otters. 531 pp.
Vermeulen, E., Fruet, P.F., Costa, A.P.B., Coscarella, M., Laporta, P. 2019. Tursiops truncatus ssp. gephyreus. The IUCN Red List of Threatened Species 2019: e.T134822416A135190824., 2019
Zerbini, A.N., Secchi, E., Crespo, E., Danilewicz, D. and Reeves, R. 2017. Pontoporia blainvillei (errata version published in 2018). The IUCN Red List of Threatened Species 2017: e.T17978A123792204. Available at: https://dx.doi.org/10.2305/IUCN.UK.20173.RLTS.T17978A50371075.en.